

Pfizer Inc. 400 Webro Road Parsippany - New Jersey - 07054 USA
Pfizer AG, Schärenmoosstrasse 99
8052 Zürich
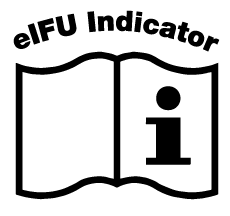
A paper copy of IFU can be obtained from the following address:
Pfizer AG, Schärenmoosstrasse 99, 8052 Zürich
Version
Acrodat ver 1.3.0
Adverse Event Reporting
Adverse Events or incidents/near incident with a medical device MUST BE reported to the EU Authorised Representative and the country Competent Authority where the event has taken place. Swissmedic (local Competent Authority) provides a simple report form (German: MU510_00_002d_FO_Medizinprodukte Vigilance Meldung Anwender, French: MU510_00_002f_FO Annonce d’incident survenue avec un dispositive medical or Italian: MU510_00_002i_FO Notifica incidente dispositivi medici) and respective information on its website (www.swissmedic.ch/md-materiovigilance-user).
Swissmedic, Swiss Agency for Therapeutic Products
Medical Devices Department
Hallerstrasse 7
CH-3012 Bern
Tel. +41 58 463 22 51, Fax +41 58 462 76 46
E-Mail: materiovigilance@swissmedic.ch
Adverse Events or incidents/near incident related to a Pfizer product should be reported to Pfizer pharmacovigilance department using the following link: German: Unerwünschte Arzneimittelwirkung / Produktbeanstandung melden | Pfizer Switzerland French: Notification D'effet Indésirable / Réclamation Concernant Un Produit | Pfizer Switzerland


